Need peace of mind for sterilisation? A Vacuum Autoclave is the best choice for veterinary practices! Steam sterilisation is efficient, destroying microbes fast. Unlike non-vacuum types, the vacuum forcibly...
See our portable DR radiography in action at Bristol Zoo Project! Witness how the dedicated vet team and keepers use positive reinforcement training to get willing participation from animals like Dayo...
By Joel Huey - Content Editor & Dan Williams - Project Manager
Do you know what to look out for when researching piped gas systems?
There are lots of articles that tell you what materials are used, but...
The first thing you notice when you walk into the Big Cat Sanctuary is the warmth of the people that work there. Not just towards the visitors supporting such a great cause, but towards the resident cats...
By Courtney Scales DipVN NCert (Anaesth) RVN
Airway devices are used to secure and maintain and protect a patent airway, provide a means to deliver oxygen and volatile gases, remove expired waste gases...
By Joel Huey BA (HONS) - Content Editor
Read time: 2 minutes
When selecting a bur to purchase, you need to have the answers to the following two questions:
- 1. What shank does my handpiece require?
- 2. What...
In today's Clinical Tip video, Courtney, our Clinical Educator, will show you how to attach a couple of IPPV accessories to your circuit. These will allow you to safely deliver a positive pressure breath...
The AG Cuffill is a cuff inflator and manometer, suitable for use with several airway devices; ETTs, tracheostomy tubes, and laryngeal mask airways, including supraglottic airway devices.
To get one for...
In today's video, Keith Simpson, our Clinical Educator will be running through some tests you can try in practice to ensure that your Merlin Ventilator is running smoothly. He also shows you the initial...
In today's video, Courtney, our Clinical Educator, will be showing you how to leak test the anaesthetic machine and common breathing systems you have in practice. If there are any cracks or leaks in the...
In today's video our Clinical Director, Keith Simpson will be testing your Circle Circuit for compatibility with the Merlin Small Animal Ventilator...
Keith will show you how to test the compatibility...
"In today's video, Courtney, our Clinical Educator, will be showing you how to test the ECG cables on your ECG machine. If there is a problem suspected with the ECG cable or electrode, several tests can...
Our Clinical Educator, Courtney Scales is introducing the HotDog Veterinary Warming System.
The HotDog Veterinary Temperature Management Controllers have been developed with user experience and functionality...
In today's video, Courtney, our Clinical Educator will be explaining the different aspects of Hypothermia, and why it is one of the most common complications in a vet practice.
Learn more on our heat mats...
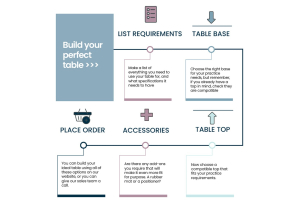
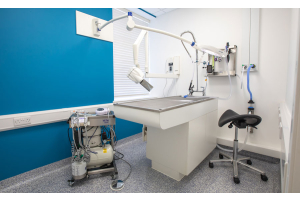
There is a vast range of Burtons manufactured tops and bases, from standard consults to operating tables with positioners. We know that it can seem a daunting number of options, so let us guide you through...
Dental services can be one of the most cost-efficient departments of a veterinary practice, and therefore making sure you have long-lasting, cost-effective equipment to hand is a must. In this guide, we...
If you have already read our article on ‘How to choose your multi-parameter monitor’ you will be aware of the key considerations for purchasing a new monitor, and the difference it can make having a...
The two most used types of radiography in veterinary practices are CR – computed radiography, and DR - Direct radiography. Both options are a huge developmental jump from film radiography, where you had...
Burtons ‘Lifetime’ Cages
Perfect all-rounders for a large variety of patients
Fully Configurable
Constructed from high grade stainless steel
Designed to Last a 'Lifetime'
Hardwearing and easy to clean,...